
Alphabetical Index
Chemical Composition
Keyword Search
Named Inclusions
Steel Index
Exogenous Inclusions
Indigenous Inclusions
Macro Inclusions
Micro Inclusions
Nano Inclusions
Iron Oxide Inclusions
Nitride Inclusions
Oxide Inclusions
Phosphide Inclusions
Silicate Inclusions
Spinel Inclusions
Sulfide Inclusions
Refractory Inclusions
Slag Inclusions
Figure Browser
Help
Contact Us
Home
The copper sulfide + iron sulfide coexisted with silicon oxide in sample H13L
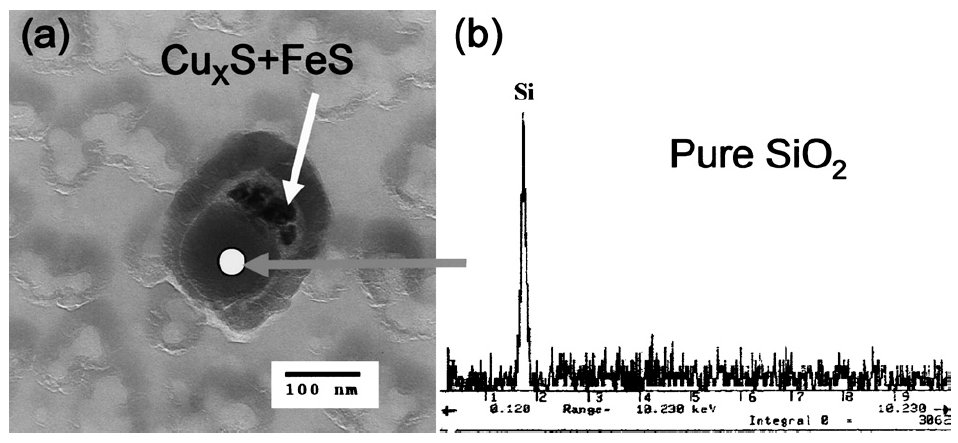
Figure 2: The tiny copper sulfide coexisted with silicon oxide (a) and the EDS spectrum for silicon oxide (b) in sample H13L (Nylon grid). Scale bar: 100 nm.
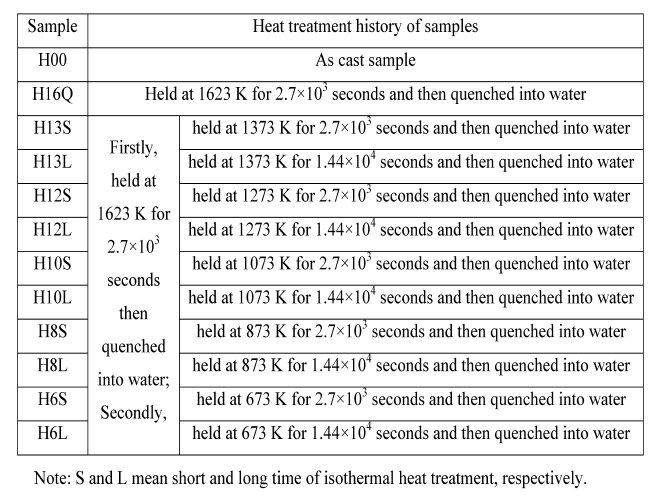
Table 1: The detail heat treatment history of samples.
Inclusion name: Copper sulfide + Iron sulfide and silicon dioxide
Record No.: 866
Inclusion formula: CuxS + FeS + SiO2
Inclusion type (Macro/Micro/Nano): Nano
Inclusion type (Exogenous/Indigenous): Indigenous
Inclusion classification: Sulfide, oxide
Inclusion composition in weight %: No data
Sample: Steel
Steel composition in weight %: 0.005% C, 0.25% Cu, 0.024% S, 0.0080% P, 0.0012% N.
Note: Copper and sulfur are typical residual elements or impurity elements in steel. Sufficient removal of them during steelmaking process is difficult for copper and costly for sulfur. Utilization of copper and sulfur in steel, especially in steel scrap, has been an important issue for a long period for metallurgists. Copper and sulfur may combine to form a copper sulfide, which may provide a prospect to avoid the detrimental effects of copper and sulfur in steel. Unfortunately the formation mechanism of a copper sulfide
in steel has not been completely clarified so far. In the present paper, solution treatment of samples containing copper and sulfur are firstly performed at 1623 K for 2.7 x exp10(3) s followed by quenching into water. The samples are then isothermally heat-treated at 673 K, 873 K, 1073 K, 1273 K and 1373 K for different time followed by quenching into water again. The size, morphology, constituent and crystallography of sulfide precipitates in these samples are investigated by SEM and TEM equipped with EDS. Fine copper sulfides (less than 100 nm) are observed to co-exist with silicon oxide in samples even isothermally heattreated at 1373 K for 1.44 x exp 10(4)s. Film-like copper sulfides are generally observed to co-exist with iron sulfide in all samples; Plate-like copper sulfides are observed especially in sample isothermally heat-treated at 1073 K for 1.44 x exp10(4) s. The formation mechanisms of these copper sulfides have been discussed in detail.
It is interesting that the oxide inclusion which coexisted with CuxS is almost pure silicon oxide, as shown in Fig. 1. It has been thought that complex silicate oxide, which has low melting point and high sulfur capacity, could nucleate
the sulfide precipitation in steel; while pure silicon oxide has no such ability since its high melting point and low sulfur
capacity. Although Holzheld has reported that copper has some solubility in silicate melts irrespective of the presence
of sulfur, there has no such report for copper in pure silicon oxide. On the other hand, Wakoh has investigated
the effect of sulfur content on the MnS precipitation with oxide nuclei in steel deoxidated by Mn–Si, Mn–Ti, Al and
Zr, respectively. It is found that the precipitation ratio of MnS on oxide is large in almost all oxides when the content
of sulfur is higher than 0.01 mass% in steel. The present precipitation of CuxS on pure silicon oxide may be also that
case. That is the pure silicon oxide works as the precipitation site of CuxS.
Additional links: Not shown in this demo version.
Reference: Not shown in this demo version.