
Alphabetical Index
Chemical Composition
Keyword Search
Named Inclusions
Steel Index
Exogenous Inclusions
Indigenous Inclusions
Macro Inclusions
Micro Inclusions
Nano Inclusions
Iron Oxide Inclusions
Nitride Inclusions
Oxide Inclusions
Phosphide Inclusions
Silicate Inclusions
Spinel Inclusions
Sulfide Inclusions
Refractory Inclusions
Slag Inclusions
Figure Browser
Help
Contact Us
Home
Manganese Sulfide
Chemical formula: MnS
Modifications: Two intermediate phases, MnS and MnS2 have been reported for Mn-S system. Three different modifications of MnS are known, alpha, beta and beta'. MnS also forms a mineral, hauerite.
Melting point: 1610oC
Density: 3.99 g/cm3
Microhardnes: 170 kp/mm2
Hardness (Moh's): 2-2.5
Colour: The colour of inclusions of the phase in situ is light grey or blue gray in the optical microscope, it is isotropic and usually slightly transparent.
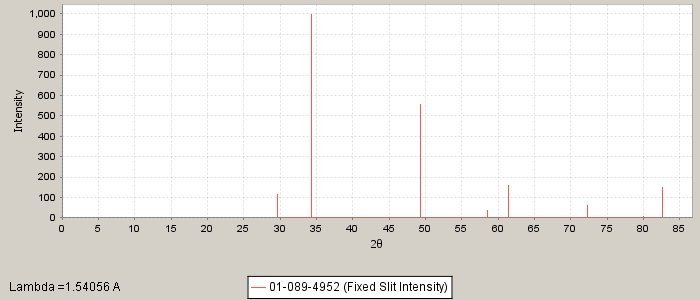
Crystal system: Cubic
Cell dimensions: a=5.2226 A
PDF number: 01-089-4952
ICSD number: Data not found
Note: Inclusions of the MnS-type are the most frequent and also most important sulfide inclusions in modern steel. Substiyutional solid solution of the alpha-MnS are known, where Mn is substituted by other metals. Double sulfides of the spinel type with the formula MnS x B2S2 are known. Lead in insoluble in MnS, but morphology of the MnS phase seems to be influenced by this metal. MnS inclusions with lead tips have, for instance, been observed in free-machining steels. MnS as an inclusion phase is easily recognized in the optical microscope. It is light-grey, plastic, slightly transparent and inactive to polarized light. Deformation of the steel usually results in the MnS-phase being elongated in the working direction, forming strings or plates. The morphology of MnS in steel ingots depends on the steelmaking practice. Using the classification given by Sims and Dahle (1938), the sulphide phase may be desribed as a type I, type II pr type III sulfide. Type I is globular with wide range of sizes, often duplex with oxygen compounds and with an apparently random distribution. This type is most common in rimmed steel, where Si has been the main deoxidant and is found in carbon as well as in alloyed steels. Duplex inclusions of type I with MnS and silicates may vary in appearance depending on the sulphur to oxygen ratio. The MnS phase in this inclusion type often has varying ammounts of other elements, for example Cr. MnS type II has a dendritic structure and is often called grain-boundary sulfide because it is distributed as chainlike formations or this precipitates in primary ingot grain boundares. Sometimes it seems to have solidified in a eutectic pattern, and it is often found crystallized together with corundum. This type is found in steels, deoxidized with Al without an excess of the deoxidizing metal. Type III is irregular, often angular in shape and randomly distributed in the steel. This type is often similar to type I and there is no marked difference between the two types. However type III always forms monophase inclusions whereas type I usually is present in multiphase inclusions. Type III is most frequently found in steels deoxidized with an excess of Al.