
Alphabetical Index
Chemical Composition
Keyword Search
Named Inclusions
Steel Index
Exogenous Inclusions
Indigenous Inclusions
Macro Inclusions
Micro Inclusions
Nano Inclusions
Iron Oxide Inclusions
Nitride Inclusions
Oxide Inclusions
Phosphide Inclusions
Silicate Inclusions
Spinel Inclusions
Sulfide Inclusions
Refractory Inclusions
Slag Inclusions
Figure Browser
Help
Contact Us
Home
A typical calcium aluminate inclusion surrounded with a CaS ring observed in a high carbon Si killed low aluminum steel
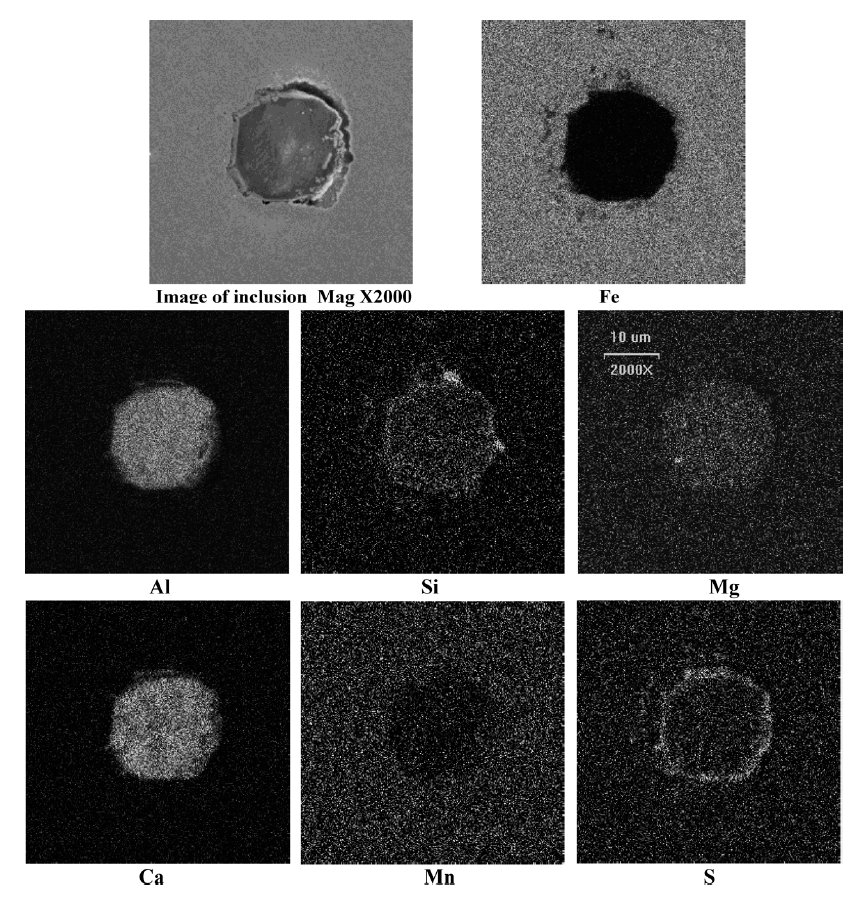
Figure 1: A typical calcium aluminate inclusion surrounded with a CaS ring observed in a high carbon Si (0.17 mass%)
killed low aluminum (0.005 mass%) steel sample after calcium treatment in the ladle at 1823 K. SEM, scale bar: 10 µm.
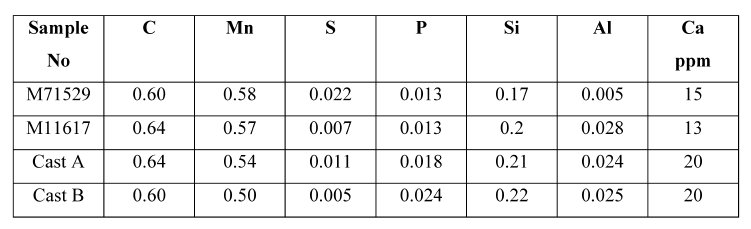
Table 1: Chemical composition (mass%) of steel samples.
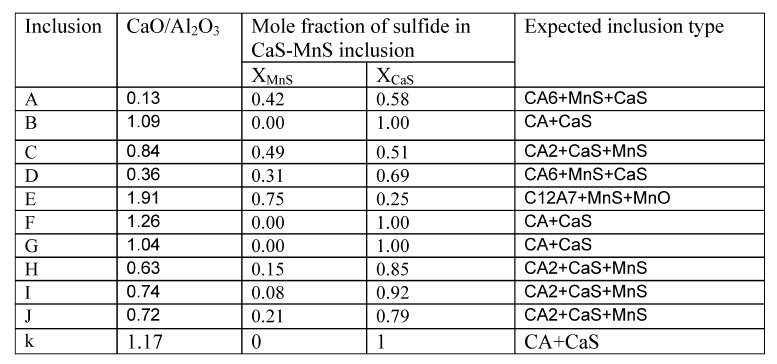
Table 2: Characteristics of inclusions observed in steel samples.
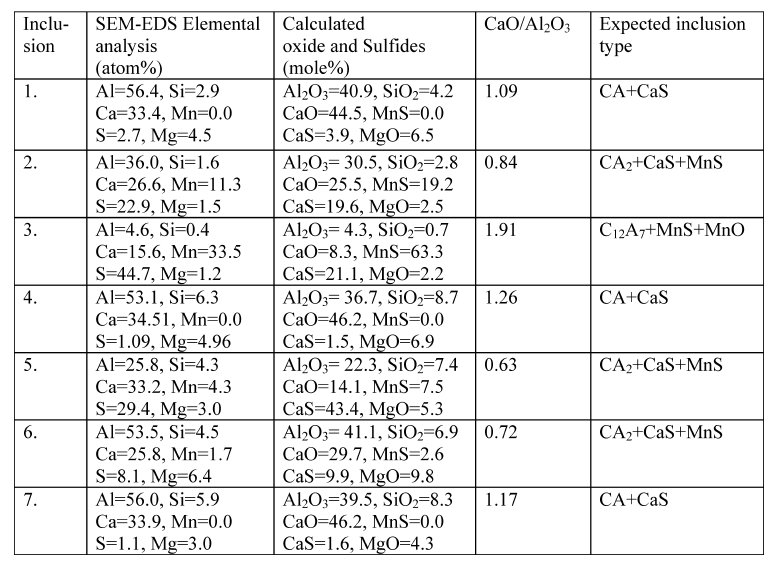
Table 3: Composition of some of the representative inclusions observed in steel samples.
Inclusion name: Calcium aluminate
Record No.: 1115
Inclusion formula: See the table
Inclusion type (Macro/Micro/Nano): Micro
Inclusion type (Exogenous/Indigenous): Indigenous
Inclusion classification: Oxide
Inclusion composition in weight %: See the tables 2 and 3
Sample: Calcium treated Al-killed steel
Steel composition in weight %: See the table 1.
Note: Calcium is widely used for improving the castability of liquid steel, as well as for improvement of steel
cleanliness and inclusion modification for better quality steel. Calcium modifies solid alumina inclusions,
arising out of deoxidation of liquid steel, into liquid calcium aluminate. Depending upon the steel composition,
calcium sulfide (CaS) and/or various forms of calcium aluminates may form. Sulfides are often associated
with the oxide phase, which is typically known as oxide–sulfide duplex inclusion. Formation of solid calcium
sulfide must be avoided during the ladle treatment of liquid steel, since it is detrimental to the castability
of steel. In the present work a thermodynamic model has been developed for predicting the formation of
oxide–sulfide duplex inclusions arising out of competitive reactions between [O], [S] and [Ca] in Al-killed
steel. The model predictions of the present work were compared with those reported in literature, as well
as with the types of inclusions observed in steel samples collected from the plant. Reasonably good agreements
amongst them were observed. The results indicated that in order to achieve completely liquid calcium
aluminate without forming any sulfides the sulfur content of liquid steel must be sufficiently low. With
increasing S content of liquid steel, complete modification of alumina inclusions into liquid calcium aluminate
becomes difficult. The maximum sulfur content to avoid formation of CaS depends upon the steel
composition, principally aluminum. The sulfide inclusions are often a solid solution of CaS and MnS. Thermodynamic
analysis for this system was also carried out.
Most of the inclusions in steel samples were found to be quite small in size (<6 µm). Mostly, inclusions had a calcium aluminate core surrounded with a sulfide ring of
CaS–MnS or CaS. A typical oxide–sulfide duplex inclusion observed in liquid steel sample after calcium treatment is shown in Fig. 1. X-ray map of the constituent elements of
the same is also shown in top Fig.. Chemical compositions of inclusions were determined from the EDS. These are noted in tables.
Reference: Not shown in this demo version.